
IRXross
IRXross FTIR spectrophotometer -Xrossing Performance x Operability-
The IRXross Fourier Transform Infrared (FTIR) Spectrophotometer adds the new versatility to the world of infrared spectroscopy, combining high performance with operational ease to meet the demands of the pharmaceutical and chemical industries. Boasting the best-in class S/N ratio of 55,000:1, along with high resolution and measurement speeds that rival the larger models, the IRXross offers the optimal solution for diverse application requirements.
This model includes the IR Pilot analysis support program, ensuring accurate measurement results can be easily obtained even by users with limited analysis experience. It is also compliant with data integrity regulations required in the pharmaceutical field.
Product Description
IRXross FTIR spectrophotometer -Xrossing Performance x Operability-
The IRXross Fourier Transform Infrared (FTIR) Spectrophotometer adds the new versatility to the world of infrared spectroscopy, combining high performance with operational ease to meet the demands of the pharmaceutical and chemical industries. Boasting the best-in class S/N ratio of 55,000:1, along with high resolution and measurement speeds that rival the larger models, the IRXross offers the optimal solution for diverse application requirements.
This model includes the IR Pilot analysis support program, ensuring accurate measurement results can be easily obtained even by users with limited analysis experience. It is also compliant with data integrity regulations required in the pharmaceutical field.
Technical specifications
High-End Sensitivity for Countless Applications
The IRXross is a mid-level FTIR model that achieves high-end level S/N. It enables best-in-class low noise with P-P values of 55,000:1 for one minute of integration.
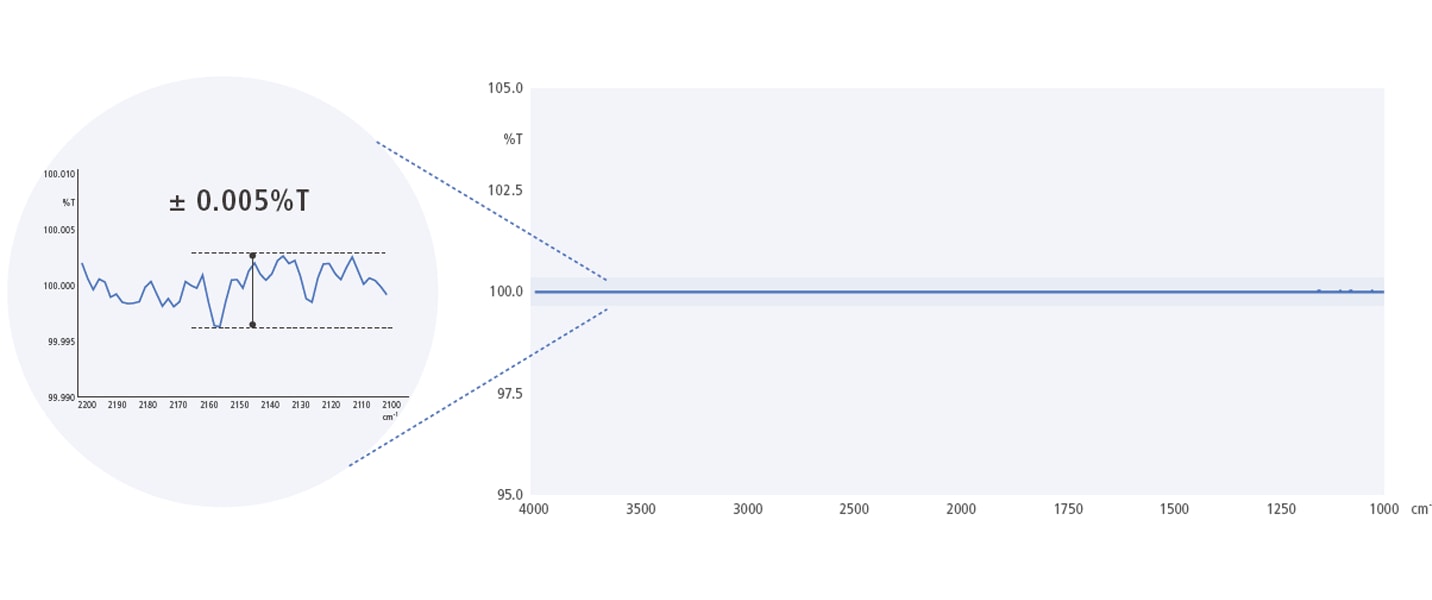
Astoundingly Low Noise
The 100 %T line was obtained by successively measuring the background and sample values without placing a sample in the sample compartment. Excluding the peaks for water vapor and carbon dioxide, the noise level (P-P value) was within ±0.005 %T, which shows that it can acquire data with low noise.
Built- in Analytical Intel l igence
-
Easy Navigation with IR Pilot™ Ensures Anyone Can Get Started Easily
A total of 23 macro application programs are included. Even operators unfamiliar with FTIR analysis can analyze samples easily by simply selecting the purpose of analysis and attachment used. There is no need to set parameters. Multiple samples can be analyzed with a single click.
Complies Fully with Regulations
Humidity-Resistant Window Material Compatible with the Wavenumber Measurement Range Specified in Pharmacopoeia
Either a KBr or KRS-5 window can be selected. The KRS-5 window maintains humidity resistance up to 90 %RH (for temperatures up to 30 °C) and is compliant with pharmacopoeia wavenumber range requirements (350 to 7,800 cm-1).
Data Integrity Compliance
Solid Security
An audit trail to ensure the reliability of data and document e-mail transmission functions when any event occurs in the system can be set up. User accounts are managed using passwords, where password length, complexity, and term of validity must satisfy specified requirements. It is also possible to set lockout functions to prevent illegal access and set a registered user’s deletion and change in status. In addition, a box can be selected to prevent overwriting a data file, and outputting an item to a report can be performed.